Abstract
Background
Hemophilia is a rare bleeding disorder characterized by deficiency of FVIII or FIX resulting in ineffective clot formation due to impaired thrombin generation. Fitusiran is a subcutaneously (SC) administered investigational siRNA therapeutic agent which targets antithrombin to enhance thrombin generation potential and rebalance hemostasis in people with hemophilia (PwH), irrespective of inhibitor status. Here, we present results from a Phase 3 study of the efficacy and safety of fitusiran prophylaxis compared with on-demand (OD) treatment with factor concentrates in PwH A or B without inhibitors (ATLAS-A/B; NCT03417245).
Methods
This Phase 3, multicenter, multinational, randomized, open-label study evaluated the efficacy and safety of fitusiran in males aged ≥12 years with severe hemophilia A or B without inhibitors, previously treated OD. Eligible participants (pts) were randomized 2:1 to receive either once-monthly 80 mg SC fitusiran prophylaxis (fitusiran arm) or OD factor concentrates for treatment of bleeding episodes (OD arm) for a treatment period of 9 months. The primary endpoint was annualized bleeding rate (ABR) in the efficacy period (Day 29 post-first fitusiran dose up to Day 246). Secondary endpoints included annualized spontaneous bleeding rate (AsBR) and annualized joint bleeding rate (AJBR) in the efficacy period and health-related quality of life (HRQoL) in the treatment period, measured by Haem-A-QoL. Safety and tolerability were assessed throughout the study.
Results
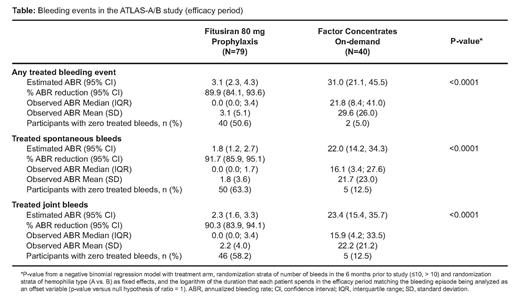
Overall, 120 pts were randomized (fitusiran arm n=80; OD arm n=40); 79 pts (98.8%) in the fitusiran arm and 37 pts (92.5%) in the OD arm completed the study. A total of 93 pts had hemophilia A (fitusiran arm n=62, OD arm n=31) and 27 pts had hemophilia B (fitusiran arm n=18, OD arm n=9). Baseline demographics and characteristics were similar in both arms. Median observed ABR was 0.0 (IQR, 0.0 to 3.4) in the fitusiran arm and 21.8 (IQR, 8.4 to 41.0) in the OD arm; 40 pts (50.6%) in the fitusiran arm experienced no bleeds that required treatment with OD factor concentrates. A significant reduction in estimated ABR was achieved in the fitusiran arm vs the OD arm (89.9% reduction [95% CI, 84.1% to 93.6%], p<0.0001; Table). Median observed AsBR was 0.0 (IQR, 0.0 to 1.7) in the fitusiran arm and 16.1 (IQR, 3.4 to 27.6) in the OD arm. A significant reduction in estimated AsBR was achieved in the fitusiran arm vs the OD arm (91.7% reduction [95% CI, 85.9% to 95.1%], p<0.0001; Table). Median observed AJBR was 0.0 (IQR, 0.0 to 3.4) in the fitusiran arm and 15.9 (IQR, 4.2 to 33.5) in the OD arm. There was a significant reduction in estimated AJBR in the fitusiran arm vs the OD arm (90.3% reduction [95% CI, 83.9% to 94.1%], p<0.0001; Table). A significant improvement was also achieved in the transformed total and physical health score in the fitusiran arm vs the OD arm (LS mean difference -7.07 [95% CI, -11.23 to -2.90], p=0.0011; -19.75 [95% CI, -27.00 to -12.50], p<0.0001, respectively).
Seventy-nine pts received at least 1 dose of fitusiran and 40 patients were randomized to the OD arm and included in the safety analysis. Overall, 62 pts (78.5%) in the fitusiran arm and 18 pts (45%) in the OD arm experienced ≥1 treatment emergent adverse event (TEAE). A total of 5 treatment emergent serious adverse events (TESAEs) were reported in 5 pts (6.3%) in the fitusiran arm and 9 TESAEs were reported in 5 pts (12.5%) in the OD arm. TESAEs in the fitusiran arm included cholelithiasis (2 pts, 2.5%), cholecystitis, lower respiratory tract infection, and asthma (1 pt each, 1.3%). In the fitusiran arm, 2 pts (2.5%) experienced TEAEs that resulted in fitusiran discontinuation (cholecystitis and increased alanine aminotransferase). No TEAEs of thrombosis and no fatal TEAEs were reported.
Conclusions
All key primary and secondary endpoints were met in this Phase 3 study. Specifically, once-monthly 80 mg SC fitusiran prophylaxis demonstrated a significant reduction in ABR, AsBR and AJBR (all ~90%) in people with severe hemophilia A or B without inhibitors compared with OD treatment. This reduction in bleeding was associated with a meaningful improvement in HRQoL. Reported TESAEs were generally consistent with previously identified risks of fitusiran. With the aim of further enhancing the benefit-risk profile of fitusiran, a revised regimen with reduced dose and frequency is currently being evaluated in ongoing clinical studies.
Srivastava: Roche: Other: Advisory Board, Research Funding; Novo Nordisk: Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board, Research Funding; Pfizer: Other: Advisory Board, Research Funding; Takeda: Other: Advisory Board, Research Funding; Bayer Healthcare: Other: Grant Review & Awards Committee. Rangarajan: Sanofi: Other: Advisory Board; Pfizer: Other: Advisory Board; Reliance Life Sciences: Consultancy; Takeda: Other: Advisory Board, Conference Support, Speakers Bureau. Kavakli: Pfizer, Bayer, Takeda, Roche, Novo Nordisk: Honoraria; Pfizer, Bayer, Roche, Novo Nordisk, Takeda: Speakers Bureau; Roche, Bayer, Pfizer, Novo Nordisk, Takeda: Membership on an entity's Board of Directors or advisory committees. Klamroth: Bayer, Leo: Research Funding; Bayer, Biotest, Biomarin, BMS, CSL Behring, Daiichi Sankyo, Leo, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, SOBI, Takeda: Honoraria; Bayer, Biotest, Biomarin, BMS, CSL Behring, Daiichi Sankyo, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, SOBI, Takeda: Speakers Bureau. Kenet: Alnylam: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Opko Biologics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Shire: Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding; Nat'l Hemophilia Ctr and Coagulation Unit and Amalia Biron Res Inst of Thromb & Hemost, Sheba Medical Ctr, Tel Hashomer, Israel: Current Employment; BioMarin Pharmaceutical: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Uniquore: Honoraria, Membership on an entity's Board of Directors or advisory committees; BPL: Research Funding. Khoo: NSW Health Pathology: Current Employment; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau. You: Eulji University Hospital: Current Employment. Malan: PHOENIX Pharma (Pty) Ltd, Qgeberha, South Africa: Current Employment, Research Funding; St Francis Hospital, Qgeberha, South Africa: Membership on an entity's Board of Directors or advisory committees. Frenzel: Pfizer: Research Funding; Roche: Research Funding; SOBI: Research Funding; CSL Behring: Research Funding. Stasyshyn: CSL Behring: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Research Funding, Speakers Bureau; Octapharma: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; Shire: Consultancy, Honoraria, Research Funding; Institute of Blood Pathology and Transfusion Medicine of National Academy of Medical Sciences of Ukraine: Current Employment; Sanofi: Honoraria, Research Funding; Roche: Speakers Bureau; LFB: Honoraria, Research Funding. Poloskey: Sanofi: Current Employment, Current equity holder in publicly-traded company. Andersson: Sanofi: Current Employment, Current equity holder in publicly-traded company; WEST advisory committee member: Membership on an entity's Board of Directors or advisory committees. Mei: Sanofi: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Pipe: Sangamo Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board; ASC Therapeutics: Consultancy, Other: Scientific Advisory Board; Apcintex: Consultancy; Bayer: Consultancy; Biomarin: Consultancy; Catalyst Biosciences: Consultancy; CSL Behring: Consultancy; Freeline: Consultancy; Grifols: Consultancy; HEMA Biologics: Consultancy; Novo Nordisk: Consultancy; Octapharma: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; Spark Therapeutics: Consultancy; uniQure: Consultancy; GeneVentiv: Consultancy, Membership on an entity's Board of Directors or advisory committees; YewSavin: Research Funding; Siemens: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal